Is Carbon Disulfide Soluble in Water
An Introduction to Carbon Disulfide. Colorless liquid bp 46 C mp -111 C Slightly soluble in water 022 g100 mL Odor.
Miscible with ethanol ether.

. Siodine i2 more soluble in water or in carbon disulfide cs2. CS2 is highly flammable. Raymond Chang Jason Overby Rent Buy.
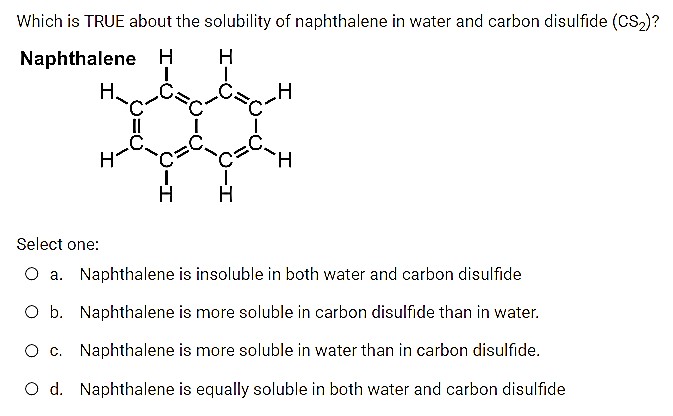
Although its soluble in many organic solvents like benzene chloroform etc. Naphthalene is more soluble in carbon disulfide than in water. Was this answer helpful.
Best Answer Copy Carbon disulfide is not soluble in water. 18 Pure carbon disulfide has a sweet pleasant chloroform-like. As a result it sinks in water since vapors are heavier than air.
However it has low reactivity at low temperatures. While however reactions of nucleophileswith CO2 are highly reversible and products are only isolated with very. Water-insoluble and denser 105 lbgal than water.
Pure carbon disulfide is a colorless liquid with a pleasant odor that is like the smell of chloroform. Carbon disulfide evaporates at room temperature and the vapor is more. Sulphur is insoluble in water ether and alcohol.
Soluble in water. Phosphorus sulfur selenium bromine iodine lipids resins rubber and asphalt are all soluble in carbon disulfide. Option C is carbon disulphide.
Caros acid H2SO5 with an electrochemical oxidizing potential of 254V might do the job or else aqua regia mixture of nitric and hydrochloric acids. Carbon disulfide CAS RN. Carbon disulfide is not soluble in water.
Impure carbon disulfide is yellowish. Carbon disulphide is a non-polar solvent. According to like dissolve like rule sulphur is soluble in carbon disulphide.
It is soluble in carbon disulfide and in other nonpolar organic solvents such as benzene and toluene. Is iodine I2 more soluble in water or in carbon disulfide CS2. Some applications of sulphur are Sulfur is used as a fungicide and in black.
This is aided by the fact that its heavier than water and is not miscible in it. What will happen during cooling. Slightly soluble in water.
Carbon disulfide Carbon bisulfide CAS 75-15-0. Thus option C is the correct answer. Linear molecule with double bonds between the sulfur atoms and the central carbon atom.
2 Show answers Another question on Chemistry. General Chemistry 6th Edition Edit edition Solutions for Chapter 13 Problem 1PE. Which one of the following statements is correct when S O 2.
Akno3 solution containing 51 g of kno3 per 1000 g of water is cooled from 40 c to 0 c. Pure carbon disulfide occurs as a colorless liquid that is not very soluble in water. Which is TRUE about the solubility of naphthalene in water and carbon disulfide CS2 Naphthalene H H H.
Handbook of Aqueous Solubility Data Second Edition. Single-walled carbon nanotubes have been purified using this method. Hence CS 2 is always stored under a layer of water.
Hawleys Condensed Chemical Dictionary 16th Edition Soluble in chloroform. A salt effect of up to 25 is observed on dissolution in 05 molar NaCl or coastal seawater. Carbon disulfide evaporates rapidly at room temperature and is flammable.
I2 solubility in water is 0029 g per 100 cm3 at 20 C Carbon disulfide 022 g per 100 cm3 Would you like to suggest why CS2 is more soluble. Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other nonpolar organic solvents such as benzene and toluene. But these reactions are going to yield CO andor CO2 which can dissolve in water.
Carbon disulphide and water are two immiscible solvents and iodine is more soluble in carbon disulphide than in wateris it possible to extract out iodine completely from its aquous solution by use of carbon disulphidegive a comment. We know sulphur and most of its compounds are nonpolar. In water 2160 mgL at 25 deg C.
CS2 3 O2 CO2 2 SO2Compared to the isoelectronic carbon dioxide CS2 is a weaker electrophile. Naphthalene is more soluble in water than in carbon disulfide. Carbon disulfide is a linear molecule with double bonds between the sulfur atoms and.
Naphthalene is insoluble in both water and carbon disulfide O b. Cabbage-like odor detectable at 0016 to 042 ppm mean 02 ppm Vapor Density. Carbon disulfide is prepared by heating sulfur and charcoal.
The impure carbon disulfide that is usually used in most industrial processes is a yellowish liquid with an unpleasant odor like that of rotting radishes. Although the electronegativities of carbon and sulfur are similar. Since option C is the correct answer Option D is also the wrong answer.
Probably the only way youre going to dissolve carbon in a solvent is by reacting with a strong oxidizer. At normal temperature carbon. The solubility of carbon disulfide in water expressed as the dimensionless ratio of concentration in the vapor phase to that in the liquid lies between 02 and 10 over the surface oceanographie temperature range 032C for an aqueous phase concentration of 5 10 4 molar.
Its combustion affords sulfur dioxide according to this ideal stoichiometry. 26 air 10 Vapor Pressure 300 mmHg at 20 C.

Solved Carbon Disulfide Cs2 And Water H20 Are Liquids Chegg Com

Solved Which Of The Following Compounds Will Be Most Soluble Chegg Com

No comments for "Is Carbon Disulfide Soluble in Water"
Post a Comment